Combined molecular docking, homology modelling and density functional theory studies to modify dioxygenase to efficiently degrade aromatic hydrocarbons†
Abstract
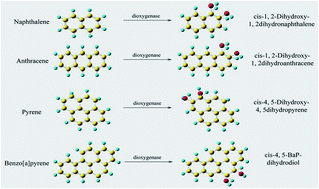
To promote the biodegradation of aromatic hydrocarbons in petroleum-contaminated soils, naphthalene dioxygenase (NDO), which is the key metabolic enzyme that degrades aromatic hydrocarbons, was modified using molecular docking and homology modelling. The novel NDO enzymes screened can efficiently degrade the target aromatic hydrocarbons naphthalene, anthracene, pyrene and benzo[a]pyrene. The docking showed that the key amino acid residues at the binding site of the NDO enzyme include both hydrophilic residues (Asn201, Asp205, His208, His213, His295 and Asn297) and hydrophobic residues (Phe202, Ala206, Val209, Leu307, Phe352 and Trp358), and the hydrophilic residues were replaced by hydrophobic residues to design 54 kinds of NDO enzyme modification schemes. A total of 14 kinds of novel NDO enzymes designed were found to simultaneously increase the binding affinity to the target aromatic hydrocarbons. The energy barrier and rate constant of the degradation reaction for the NDO enzyme modification were calculated using Gaussian09 software and the KiSThelP program. The novel NDO-7 enzyme exhibited decreases in the energy barrier of 76.28, 26.35, 4.39 and 1.88 kcal mol−1 and increases in the rate constant of 54, 18, 12 and 5 orders of magnitude in the degradation reactions with naphthalene, anthracene, pyrene and benzo[a]pyrene, respectively. These results provide a theoretical basis for the efficient degradation of aromatic hydrocarbons and the modification of their key metabolic enzymes.


 Please wait while we load your content...
Please wait while we load your content...