Fabrication of graphite via electrochemical conversion of CO2 in a CaCl2 based molten salt at a relatively low temperature†
Abstract
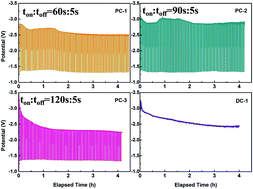
Fabrication of graphite by electrochemical splitting of CO2 in a CaCl2 molten salt is a promising approach for the efficient and economical utilization of CO2. Systematically understanding the graphitization mechanism is of great significance to optimize the process. In this work, how pulse parameter and type of anode affect morphologies and crystallinity of graphite nanostructures were both investigated. The results indicate that the optimum current efficiency, energy consumption and highest degree of graphitization can be achieved by employing an appropriate pulse current parameter (Ton : Toff = 120 : 5), and with the utilization of a RuO2–TiO2 inert anode. The microstructure and morphologies show noticeable change by varying electrolytic conditions. In addition, the present study provides an insight into facile ways to improve the graphitization degree by electrochemical conversion of CO2 at a relatively low temperature.


 Please wait while we load your content...
Please wait while we load your content...