Comparative research on three types of MIL-101(Cr)-SO3H for esterification of cyclohexene with formic acid
Abstract
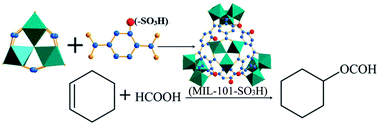
MIL-101(Cr)-SO3H was prepared by a one-pot synthesis method using CrO3 or Cr(NO3)3·9H2O as a Cr source and 2-sulfoterephthalic acid monosodium salt as a ligand with three different mineralizers, HCl, HF and NaAC, respectively. Among the prepared catalysts, MIL-101(Cr)-SO3H, which uses HCl as a mineralizer, has a high specific surface area and the strongest acidity compared with the other two mineralizers. When these catalysts were used to catalyze the esterification of cyclohexene with formic acid, MIL-101(Cr)-SO3H prepared using HCl as a mineralizer possessed the highest catalytic activity in the esterification, because the conversion rate of cyclohexene is 63.97%, whereas MIL-101(Cr)-SO3H prepared using NaAC and HF as a mineralizer shows cyclohexene conversion rates of 38.40% and 32.46%, while their selectivity to cyclohexyl formate is about 97.50%. MIL-101(Cr)-SO3H with HCl as a mineralizer can be reused three times in succession without any loss of catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...