The preparation of MgO nanopowders synthesized via an improved polyacrylamide gel method
Abstract
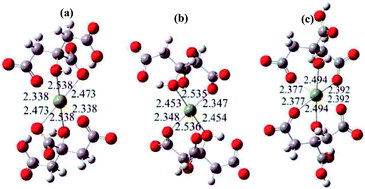
In order to address the issue of metal ion incorporation during polymerization, citric acid was used as a chelating agent to improve the polyacrylamide gel route. In the present work, MgO nanoparticles were synthesized via this improved method. The calcination temperature of the gel precursor containing magnesium nitrate was determined by thermogravimetry and differential scanning calorimetry (TG-DSC). The phases and microstructures of MgO nanopowders were identified via X-ray diffraction (XRD), transmission electron microscopy (TEM) and specific surface area measurements (BET). The results showed that the nanoparticles synthesized under 600 °C were pure, globular and about 5–20 nm in size with a narrow distribution. Furthermore, the coalescence and growth of the MgO nanograins were amazingly observed with increasing calcination temperatures and calcination time. The influence of calcination temperature on the morphology and growth behavior is greater than that of the calcination duration. The activation energy for grain growth was estimated to be 31.43 kJ mol−1, and the dominant growth mechanism was predicted to be related to the grain-rotation-induced grain coalescence (GRIGC) mechanism.


 Please wait while we load your content...
Please wait while we load your content...