Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis†
Abstract
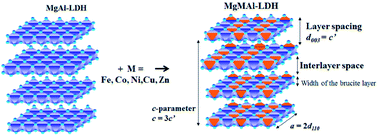
This paper details a successful synthesis and comparison of a range of tri-metal hydrotalcite-like layered double hydroxides (LDHs) using urea hydrolysis. Transition-metal-substituted MgMAl-LDHs were synthesized with M = Fe, Co, Ni, Cu or Zn. 5 mol% and 10 mol% substitutions were performed, where Mg was substituted with Co, Ni, Cu and Zn, and Al with Fe. The successful synthesis of crystalline MgMAl-LDHs was confirmed using X-ray powder diffraction (XRD) analysis. Energy-dispersive X-ray (EDX) spectroscopy was used to identify substituted metals and determine changes in composition. Changes in morphology were studied using scanning electron microscopy (SEM). Thermogravimetric analysis was used to determine the effect of Fe-, Co-, Ni-, Cu- or Zn-substitution on the thermal degradation of the MgMAl-LDH phase. The structure, morphology and thermal behavior of the LDHs were shown to be influenced by the substituted transition metals. The observed thermal stability took the order MgNiAl- > MgFeAl- = MgAl- ≥ MgCoAl- > MgCuAl- > MgZnAl-LDH. The urea hydrolysis method was shown to be a simple preparation method for well-defined crystallite structures with large hexagonal platelets and good distribution of transition metal atoms in the substituted LDHs.


 Please wait while we load your content...
Please wait while we load your content...