Differences between the binding modes of enantiomers S/R-nicotine to acetylcholinesterase
Abstract
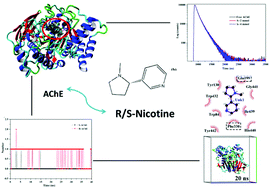
Nicotine causes neurotoxic effects because it quickly penetrates the blood–brain barrier after entering the human body. Acetylcholinesterase (AChE) is a key enzyme in the central and peripheral nervous system associated with neurotoxicity. In this study, a spectroscopic method and computer simulation were applied to explore the mode of interaction between AChE and enantiomers of nicotine (S/R-nicotine). Fluorescence spectroscopy showed that the quenching mechanism of endogenous fluorescence of AChE by S/R-nicotine was static, as confirmed by the time-resolved steady-state fluorescence. The binding strength of both nicotine to AChE was weak (S-AChE: Ka = 80.06 L mol−1, R-AChE: Ka = 173.75 L mol−1). The main driving forces of S-AChE system interaction process were van der Waals force and hydrogen bonding, whereas that of R-AChE system was electrostatic force. Computer simulations showed that there were other important forces involved. S/R-Nicotine had a major binding site on AChE, and molecular docking showed that they bound mainly to the cavities enclosed by the active sites (ES, PAS, OH, AACS, and AP) in the protein. UV-vis spectroscopy and 3D spectroscopy indicated that nicotine significantly affected the microenvironment of Trp amino acids in AChE. The CD spectra indicated that S-nicotine increased the α-helical structure of AChE, but the overall conformation did not change significantly. By contrast, R-nicotine significantly changed the secondary structure of AChE. 5,5′-Dithiobis-2-nitrobenzoic acid (DTNB) method indicated that S and R nicotine produced different degrees of inhibition on the catalytic activity of AChE. Both experimental methods and computer simulations showed that R-nicotine had a significantly higher effect on AChE than S-nicotine. This research comprehensively and systematically analyzed the mode of interaction between nicotine and AChE for neurotoxicity assessment.


 Please wait while we load your content...
Please wait while we load your content...