Au@PdAg core–shell nanotubes as advanced electrocatalysts for methanol electrooxidation in alkaline media†
Abstract
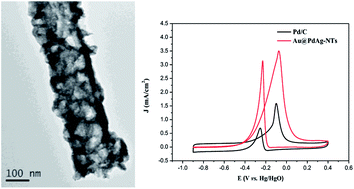
Developing active and cost-effective electrocatalysts for methanol electrooxidation is crucial to the commercialization of direct methanol fuel cells (DMFCs). In this study, Au@PdAg core–shell nanotubes are synthesized in an aqueous solution by sequential galvanic displacement between Ag nanowires and AuCl4− and PdCl42−. High-resolution transmission electron microscopy studies demonstrate that the obtained Au@PdAg nanotubes consist of a Au-rich interior that is encapsulated with a three-dimensionally dendritic, porous PdAg alloy shell, forming a core–sheath nanostructure. Electrochemical studies indicate that the as-prepared Au@PdAg nanotubes exhibit apparent electrocatalytic activity and stability towards methanol electrooxidation in alkaline media. This remarkable high performance can be attributed to their large specific surface area and unique porous morphology.


 Please wait while we load your content...
Please wait while we load your content...