Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel†
Abstract
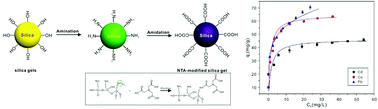
Copper (Cu2+), cadmium (Cd2+) and lead ions (Pb2+) are toxic to human beings and other organisms. In this study, a silica gel material modified with nitrilotriacetic acid (NTA-silica gel) was sensibly designed and prepared via a simple amidation procedure for the removal of Cu2+, Cd2+ and Pb2+ from water. The NTA-silica gels showed rapid removal performances for the three metal ions (Pb2+ (<2 min), Cu2+ and Cd2+ (<20 min)) with relatively high adsorption capacities (63.5, 53.14 and 76.22 mg g−1 for Cu2+, Cd2+ and Pb2+, respectively). At the same concentration of 20 mg L−1, the removal efficiencies of the three metals by the adsorbent ranged from 96% to 99%. The Freundlich and Langmuir models were utilized to fit the adsorption isotherms. The adsorption kinetics for the three metal ions was pseudo-second-order kinetics. The removal performance of the NTA-silica gels increased in a wide pH range (2–9) and maintained in the presence of competitive metal ions (Na+, Mg2+, Ca2+ and Al3+) with different concentrations. In addition, the NTA-silica gels were easily regenerated (washed with 1% HNO3) and reused for 5 cycles with high adsorption capacity. This study indicates that the NTA-silica gel is a reusable adsorbent for the rapid, convenient, and efficient removal of Cu2+, Cd2+, and Pb2+ from contaminated aquatic environments.


 Please wait while we load your content...
Please wait while we load your content...