An electrochemical sensor based on poly(procaterol hydrochloride)/carboxyl multi-walled carbon nanotube for the determination of bromhexine hydrochloride†
Abstract
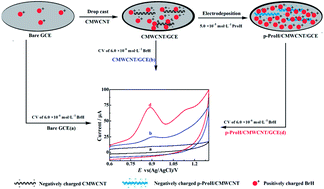
Poly(procaterol hydrochloride) (p-ProH) polymeric film was successfully deposited onto the carboxyl multi-walled carbon nanotube (CMWCNT) modified glass carbon electrode (GCE) to construct a p-ProH/CMWCNT composite modified GCE. Due to the synergistic effect of p-ProH and CMWCNT in the composite, the developed sensor can enormously enhance the oxidation peak current of bromhexine hydrochloride (BrH) at ca. + 0.90 V. Based on this appearance, an electrochemical method was established for the sensitive and selective determination of BrH with differential pulse voltammetry (DPV). Various conditions affecting the peak current response of BrH were studied and optimized. Under the best conditions, the oxidation peak current of BrH is linear to its concentration in two linear dynamic ranges of 0.2–1.0 μmol L−1 (R = 0.9948) and 1.0–8.0 μmol L−1 (R = 0.9956), with a detection limit of 0.1 μmol L−1 (S/N = 3). Interference experiment indicated that the as-prepared electrochemical sensor showed wonderful selectivity to the recognition of BrH and was free from disturbance of many other electro-active substances such as dopamine, ascorbic and uric acid. Finally, the practicability of the BrH sensor was verified by the satisfactory results acquired from the BrH determination in pharmaceutical preparation and human serum.


 Please wait while we load your content...
Please wait while we load your content...