Efficient synthesis of Ibrutinib chiral intermediate in high space-time yield by recombinant E. coli co-expressing alcohol dehydrogenase and glucose dehydrogenase†
Abstract
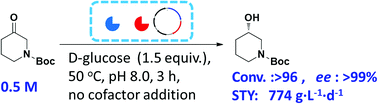
The production of (S)-N-boc-3-hydroxy piperidine (NBHP) via asymmetric bioreduction of 1-boc-3-piperidinone with reductase is impeded by the need for expensive coenzymes NAD(P)H. In order to regenerate the coenzyme in situ, the gene of alcohol dehydrogenase from Thermoanaerobacter brockii and glucose dehydrogenase from Bacillus subtilis were ligated into the multiple cloning sites of pRSFDuet-1 plasmid to construct the recombinant Escherichia BL21 (DE3) that co-expressing alcohol dehydrogenase and glucose dehydrogenase. Different culture conditions including the medium composition, inducer and pH etc were systematically investigated to improve the enzyme production. The enzyme activity was increased more than 11-fold under optimal culture condition, from 12.7 to 139.8 U L−1. In the further work, the asymmetric reduction of 1-boc-3-piperidinone by whole cells of recombinant E. coli was systematic optimized to increase the substrate concentration and reaction efficiency. At last, S-NBHP (>99% ee) was prepared at 500 mM substrate concentration without external addition of cofactors. The conversion of S-NBHP reached 96.2% within merely 3 h, corresponding a high space-time yield around 774 g L−1 d−1. All these results demonstrated the potential of recombinant E. coli BL21 (DE3) coupled expressing alcohol dehydrogenase and glucose dehydrogenase for efficient synthesis of S-NBHP.


 Please wait while we load your content...
Please wait while we load your content...