PhI(OAc)2-mediated oxidative rearrangement of allylic amides: efficient synthesis of oxazoles and β-keto amides†
Abstract
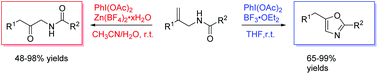
A series of 2,5-disubstituted oxazoles and β-keto amides were synthesized from allylic amides via PhI(OAc)2-mediated intramolecular cyclization and oxidation with the migration of an aryl group. These reactions were performed under mild conditions with good functional group tolerance and gave the corresponding products in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...