Ring-opening/annulation reaction of cyclopropyl ethanols: concise access to thiophene aldehydes via C–S bond formation†
Abstract
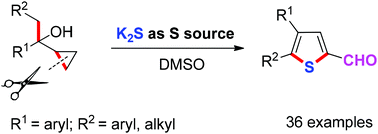
The synthesis of thiophene aldehydes from easily available cyclopropyl ethanol derivatives and potassium sulfide has been developed. This novel one-pot procedure achieves double C–S bond formation via the cleavage of C–C bonds. This transformation shows good functional group tolerance under mild reaction conditions. Moreover, DMSO acts as the solvent and mild oxidant simultaneously in this reaction.


 Please wait while we load your content...
Please wait while we load your content...