Reaction engineering and photophysical studies of fully enriched C-vinyl-1,2,3-triazoles†
Abstract
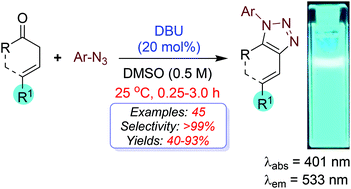
For the first time, an enolate-mediated organocatalytic fluorogenic formal [3 + 2]-cycloaddition of (E)-1,4-diarylbut-3-en-1-ones and aryl azides is reported. The mild metal-free catalytic conditions of this reaction allowed us to synthesize a library of pure fluorescent coumarin-triazole dyes. It is an efficient formal [3 + 2]-cycloaddition for the synthesis of fully decorated C-vinyl-1,2,3-triazoles with excellent outcomes with reference to the rate, yield, selectivity, operation simplicity, substrate scope, and photophysical applications.


 Please wait while we load your content...
Please wait while we load your content...