Mono epoxidation of α,ω-dienes using NBS in a water-soluble cavitand†
Abstract
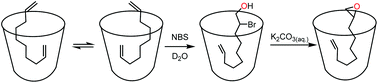
Water-soluble host molecules offer a range of environments to their guests. Polar functions of guests are exposed to the medium while hydrophobic groups are generally buried in the containers and hidden from reagents in solution. Here, we apply these preferences to convert α,ω-dienes to epoxy alkenes using cavitands as reaction vessels. Reaction of one end of a diene with NBS in water gives a bromohydrin that binds in the cavitand with the hydroxyl exposed and the remaining alkene buried. Treatment with base converts the bromohydrin to an epoxide. The reaction sequence provided up to 84% yields of monoepoxides from symmetrical dienes separated by 4 to 10 methylene groups. With 1,4-diisopropenyl benzene, a nearly quantitative yield of the monoepoxide was obtained. The application should be general for converting symmetrical hydrophobic guests to unsymmetrical, amphiphilic ones.


 Please wait while we load your content...
Please wait while we load your content...