Metal-free cascade rearrangement/radical addition/oxidative C–H annulation of propargyl alcohols with sodium sulfinates: access to 2-sulfenylindenones†
Abstract
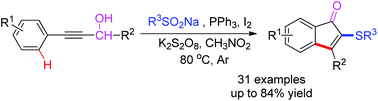
2-Sulfenylindenones were conveniently synthesized in moderate to good yields by an iodine–PPh3-mediated one-pot cascade reaction of propargyl alcohols and sodium sulfinates. The protocol may proceed through the Meyer–Schuster rearrangement of propargyl alcohols to give allenol intermediates, which is followed by the radical addition/intramolecular oxidative C–H bond cyclization sequence to afford differently substituted 2-sulfenylindenones.


 Please wait while we load your content...
Please wait while we load your content...