Synthesis of multivalent S-glycoside analogs of a heparan sulfate sequence†
Abstract
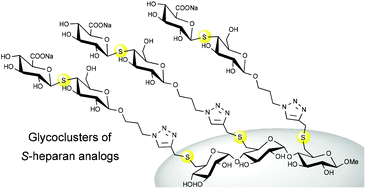
Glycosaminoglycans (GAGs) are involved in the regulation of a large number of biological processes such as inflammation, cell signalling, angiogenesis, viral infection and coagulation. Unlike molecules isolated from tissues, pure molecules, derived from organic synthesis, can prevent side effects and are very useful tools for understanding the structure–activity relationships of many biological and pharmacological activities. In our research group, we focus particularly on the synthesis of multivalent thioglycoside analogs. In this article, we report on the synthesis of new glycoclusters with thiodisaccharide units, S-analogs of heparan sulfate. The thiodisaccharide analog was obtained by nucleophilic displacement of a 4-triflate galactoside derivative, by an anomeric thiol of a glucuronic acid precursor. After modifying the aglycone part to introduce an azide, the thiodisaccharide was coupled to maltotriose scaffolds carrying one, two or three propargyl groups by CuAAC.


 Please wait while we load your content...
Please wait while we load your content...