Copper-catalyzed enantioselective Mannich reaction between N-acylpyrazoles and isatin-derived ketimines†
Abstract
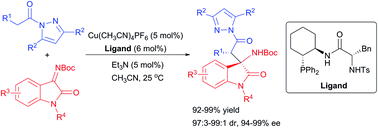
A copper-catalyzed enantioselective Mannich reaction between N-acylpyrazoles and isatin-derived ketimines is developed. With 5 mol% Cu(CH3CN)4PF6 complexed with 6 mol% chiral amidophosphine ligand L8, 3-substituted 3-amino-2-oxindoles with contiguous stereocenters were produced in 92–99% yields with excellent diastereoselectivities (97 : 3 to 99 : 1 dr) and 94–99% ee under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...