ortho-Amide-directed 2,4-dibromohydration of conjugated enynes†
Abstract
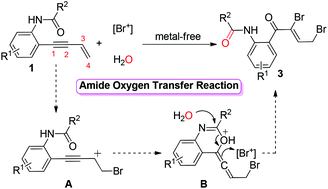
In this work, a NBS-mediated 2,4-dibromohydration of enynes is described. It has been proved that ortho-acetamide participated in this 2,4-dibromohydration process. Mechanism studies show that an amide oxygen transfers into enynes to form a carbonyl of the products. One equivalent of water is incorporated into the ortho-acetamide group. The reaction proceeds smoothly with good functional tolerance and efficiency. Interestingly, the structural elaboration is also realized when NaBH4 is employed.


 Please wait while we load your content...
Please wait while we load your content...