Visible light-mediated photo-oxygenation of arylcyclohexenes†
Abstract
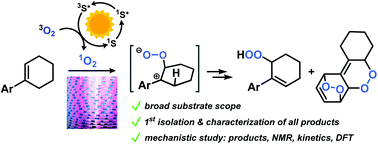
Substituted styrenes constitute important molecular building blocks for various synthetic manipulations, including selective core and peripheral oxidations. The photo-oxidation of such substrates by a singlet oxygen ene reaction (Schenck ene reaction) is especially attractive as it relies upon the combination of three abundant and cheap components: air, visible light, and an organic dye. The resultant allyl hydroperoxides enable various functionalizations to alcohols, carbonyls, epoxides, triols etc. The synthetic potential and mechanistic minutiae of photo-oxidations of styrene derivatives are not fully understood and were hitherto explored only for a very limited set of substrates. The operation of multiple oxidation events, low selectivities and yields have limited further applications of this method. Now this study reports a concise investigation of such photo-oxidations of diverse cycloalkenylbenzenes under continuous flow conditions. The combination of synthetic, kinetic, spectroscopic, and thereotical data enabled us to provide a detailed mechanistic rationalization of the observed reactivities and chemoselectivities. We propose a rarely discussed zwitterionic key intermediate of the observed allylic and [4 + 2]-dioxygenations based on a detailed Hammett study and DFT calculations. The reaction conditions of such photo-oxidations have been optimized to allow short reaction times (<10 min), high reproducibilities, and high chemoselectivities at 0 °C. Various isolation strategies of the sensitive products and their conversion to stable compounds. A set of synthetically versatile 2-aryl-2,3-epoxy-1-cyclohexanols and 1-aryl-6-hydroxy-1-cyclohexenes has been isolated and fully characterized.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...