Bifunctional Brønsted base catalyzed inverse-electron-demand aza-Diels–Alder reactions of saccharin-derived 1-azadienes with azlactones†
Abstract
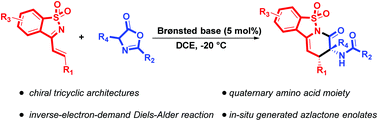
An enantioselective inverse-electron-demand aza-Diels–Alder reaction of saccharin-derived 1-azadienes and azlactones was developed using a phenylalanine-derived bifunctional Brønsted base-squaramide catalyst, which furnished the chiral tricyclic architectures bearing a quaternary amino acid moiety in high yields with moderate to excellent selectivities. A gram-scale synthesis and further elaboration of the product were also conducted to further demonstrate the synthetic utility of the reaction.


 Please wait while we load your content...
Please wait while we load your content...