Rhodium(iii)-catalyzed tandem reaction: efficient synthesis of dihydrobenzo thiadiazine 1-oxide derivatives†
Abstract
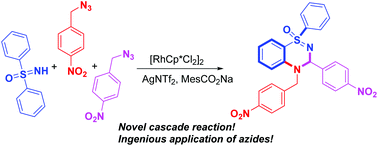
A tandem annulation reaction triggered by rhodium(III)-catalyzed C–H bond functionalizations has been well developed for highly efficient synthesis of dihydrobenzo thiadiazine 1-oxide derivatives from free NH-sulfoximine and two-component benzyl azides. This simple one-pot method generates aza-fused heterocycles which might have potential biological activities.


 Please wait while we load your content...
Please wait while we load your content...