Ruthenium(ii)-catalyzed selective C–H bond activation of imidamides and coupling with sulfoxonium ylides: an efficient approach for the synthesis of highly functional 3-ketoindoles†
Abstract
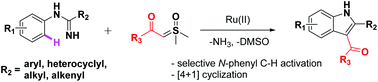
Ruthenium-catalyzed selective C–H bond activation of imidamides and annulation of sulfoxonium ylides were achieved, which afforded a series of 3-ketoindole derivatives in good yields, with good functional group compatibility. The catalytic system generated an indole scaffold by C–N and C–S bond cleavage. This reaction constitutes the first intermolecular coupling of ylides with arenes to afford a 3-ketoindole skeleton by ruthenium-catalyzed C–H activation and annulation cascade.


 Please wait while we load your content...
Please wait while we load your content...