Cp*Co(iii)-catalyzed N-alkylation of amines with secondary alcohols†
Abstract
The hydrogen borrowing methodology is a well-known, environmentally benign method for the direct alkylation of amines and alcohols as it produces only water as a side product. However, the direct alkylation of amines with secondary alcohols using first-row transition metals is very challenging. We herein report for the first time Cp*Co(III)-catalyzed direct N-alkylation of amines starting from secondary alcohols. The reaction tolerates a wide variety of functional groups, including various aryl amines and amides. Our preliminary mechanistic investigations and DFT calculations suggest that [Cp*CoI2] is an active species, that PCy3 stabilizes the high-valent hydride intermediate, and that the reaction indeed proceeds through hydrogen auto-transfer processes.
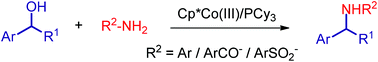


 Please wait while we load your content...
Please wait while we load your content...