Chiral phosphoric acid-catalyzed asymmetric C(sp3)–H functionalization of biomass-derived 2,5-dimethylfuran via two sequential Cope-type rearrangements†
Abstract
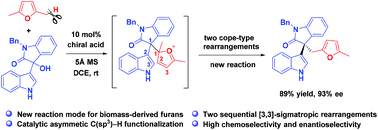
Reported herein is the first organocatalytic asymmetric C(sp3)–H functionalization of biomass-derived 2,5-dimethylfuran with 3-hydroxy-3-indolyoxindoles to afford enantioenriched furan-derived 3,3′-disubstituted oxindoles in high enantioselectivity. This reaction is realized through chiral acid-catalyzed two sequential Cope-type rearrangements as proved by DFT calculations, which demonstrates a strategically new reactivity of 2,5-dimethylfuran, enabling the direct production of chiral chemicals from biomass-derived molecules.


 Please wait while we load your content...
Please wait while we load your content...