Nickel-catalyzed regioselective C–H oxygenation: new routes for versatile C–O bond formation†
Abstract
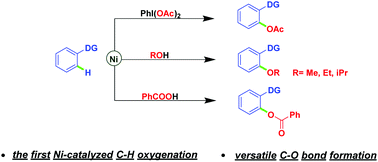
Nickel-catalyzed regioselective C–H oxygenation reactions of chelating arenes using iodobenzene diacetate, alcohols, and benzoic acids respectively as attacking reagents have been developed for the first time. Simplicity of operation, broad range of functional group tolerance, use of cheap transition metal nickel, and avoiding extraneous directing groups are the key features, thus providing an important complement to C–H oxygenation reactions and expanding the field of nickel-catalyzed C–H functionalizations. Explorations of mechanistic details are also described.


 Please wait while we load your content...
Please wait while we load your content...