Diastereoselective Rh-catalyzed decarboxylative allylation to form quaternary stereocenters using sulfinimine as the directing group†
Abstract
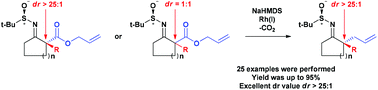
In this paper, we reported for the first time that the diastereoselective Rh-catalyzed decarboxylative allylation of chiral sulfinimines could be used to form quaternary stereocenters. The key factor in giving rise to the successful development of this method was the application of the commercially available and achiral Wilkinson's Rh catalyst. Explained by a plausible mechanism, the sulfinimine group might be a potent directing group chelated with Rh to construct intramolecular steric hindrance. In addition, broad functional group tolerance was observed, and we subsequently revealed the various transformations verifying the utility of this method for rapidly accessing complex enantio-enriched polycyclic compounds.


 Please wait while we load your content...
Please wait while we load your content...