Direct construction of 2,3-unsubstituted benzofurans and benzothiophenes via a metal-free catalyzed intramolecular Friedel–Crafts reaction†
Abstract
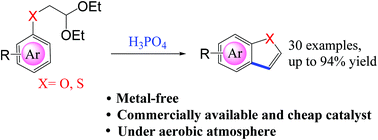
A highly effective and straightforward method for the construction of 2,3-unsubstituted benzofurans and benzothiophenes by the intramolecular Friedel–Crafts reaction has been developed. This phosphoric acid-catalyzed method exhibits good functional group tolerance for both electron-withdrawing groups and electron-donating groups. We have successfully obtained benzofuran and benzothiophene derivatives with various substituents, and the yield was up to 94%. The development of this reaction provides a simple and efficient strategy for the construction of benzofuran and benzothiophene skeleton compounds.


 Please wait while we load your content...
Please wait while we load your content...