A concise approach for determining the relative configuration of H-7 and H-8 in 8,4′-oxyneolignans by 1H NMR spectroscopy†
Abstract
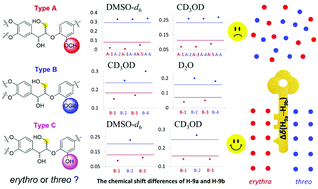
The determination of molecular configuration is usually a difficult and key point in the structure elucidation of natural products. However, due to the small number of available samples and the multiple chiral centers and complex structures of natural products, it is difficult to rapidly and accurately determine their relative configurations. In this paper, we use an NMR technique to comprehensively study 8,4′-oxyneolignan glucosides and find that the chemical shift difference between H-9a and H-9b can be used to accurately and rapidly determine the relative configuration of H-7 and H-8. Our finding can be directly applied to 8,4′-oxyneolignan glucosides without the need for hydrolysis.


 Please wait while we load your content...
Please wait while we load your content...