Cu(OTf)2-mediated C(sp2)–H arylsulfonylation of enamides via the insertion of sulfur dioxide†
Abstract
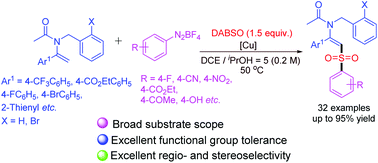
An efficient and straightforward three-component reaction of enamides, aryldiazonium tetrafluoroborates, and 1,4-diazabicyclo[2.2.2]octane bis(sulfur dioxide) (DABSO) for the direct C(sp2)–H arylsulfonylation of enamides has been developed. This Cu(OTf)2-mediated SO2 insertion reaction proceeds smoothly to afford a diverse range of β-amidovinyl sulfones bearing manifold functional groups in moderate to excellent yields with high regio- and stereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...