Metal-free cross-coupling of π-conjugated triazenes with unactivated arenes via photoactivation†
Abstract
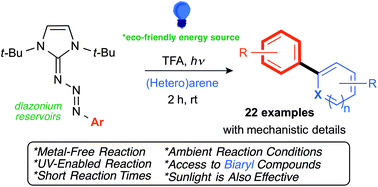
Cross-coupling of aryl compounds is one of the most powerful carbon–carbon bond forming reactions available. However, the vast majority employ scarce and expensive transition metal salts, in combination with high temperatures and long reaction times. Herein, we report a new, metal-free biaryl coupling that includes photoactivation of π-conjugated triazenes, in the presence of unactivated arenes, carried out at room temperature. Key experiments and theoretical calculations, to elucidate mechanistic details of this new, direct and significantly milder synthetic approach are presented.


 Please wait while we load your content...
Please wait while we load your content...