Supramolecular nanocatalyst in water: successive click-driven assembly of click-derived rod amphiphiles†
Abstract
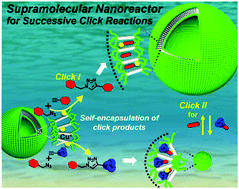
A supramolecular vesicular nanocatalyst (VC) for successive CuI-catalyzed azide–alkyne cycloaddition in water was developed by self-assembly of a click chemistry-derived rod amphiphile (CA). The CA generated by click reaction of azide-terminated benzyl ether dendrons and diethynyl naphthalene self-assembles into vesicles in water. The excellent catalytic activity of the VC was endowed by CuI-chelation to triazole-containing rod block within VC wall. The VC performed click reaction of benzyl azide with phenylacetylene or trimethylsilylacetylene. The resultant hydrophobic products self-encapsulated in VC wall affect the interfacial curvature of the VC according to their structural compatibility with CA, inducing the increase in vesicular size or structural change to micelle. Interestingly, the morphology of VC-derived nanocatalyst was further controlled from micelle to vesicle and vice versa by successive click reactions, leading to controllable loading/release of hydrophilic payloads. This recyclable catalytic activity of the self-transformable nanocatalyst was confirmed by a visually detectable click reaction offering a fluorescent color change of the aqueous VC solution.


 Please wait while we load your content...
Please wait while we load your content...