Rational design of NiFe2O4–rGO by tuning the compositional chemistry and its enhanced performance for a Li-ion battery anode†
Abstract
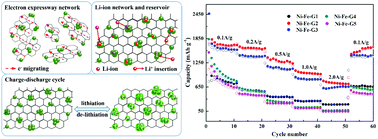
A facile in situ hydrothermal route is adopted for the synthesis of NiFe2O4–reduced graphene oxide (NiFe2O4–rGO) which is verified as a promising high-property anode material for Li-ion batteries. The formation of NiFe2O4 and the reduction of GO occur simultaneously. Ultrafine NiFe2O4 nanocrystals (below 10 nm) disperse uniformly and are immobilized by the reduced graphene oxide. The electrochemical tests show that NiFe2O4–rGO exhibits a high specific capacity of 1129 mA h g−1 at 0.2 A g−1 after ∼300 discharge–charge cycles. The excellent electrochemical properties are mainly ascribed to the introduction of the graphene matrix which offers two-dimensional conductive networks for fast electron transfer and buffers the stress from volume expansion during charge–discharge cycles. Moreover, the NiFe2O4 nanocrystals as the spacers inhibit the aggregation of rGO and form the stacking pore. More importantly, the well-designed heterostructures and the synergistic effects between NiFe2O4 and rGO can enhance the transport of Li+ and electrons and improve the structural stability of the anode, thereby enhancing the electrochemical properties. Our work not only provides an attractive strategy for the design of a hierarchical composite, but also offers a strategy for a promising anode material.


 Please wait while we load your content...
Please wait while we load your content...