Esterification of the free carboxylic group from the lutidinic acid ligand as a tool to improve the cytotoxicity of Ru(ii) complexes†
Abstract
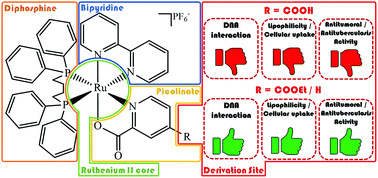
In this study, we report on the selective esterification of the carboxyl group in a coordinated ligand based on the Fischer reaction. The new [Ru(N–O)(bipy)(dppb)]PF6 complex 1 was used as a precursor to obtain the ester derivative [Ru(N–Oet)(bipy)(dppb)]PF6 (2), and in order to establish the influence of either the free carboxyl group or the ethoxycarbonyl group on biological properties, the [Ru(pic)(bipy)(dppb)]PF6 complex (3) was synthesized for comparison (dppb = 1,4-bis(diphenylphosphino)butane, bipy = 2,2′-bipyridine, N–O = mono-deprotonated 2,4-pyridinedicarboxylic acid, N–Oet = 4-ethoxycarbonyl-2-pyridinecarboxylic acid). All three complexes interact weakly with human serum albumin (HSA) with Kb values ranging from 101–104 M−1, suggesting a spontaneous interaction with this protein by electrostatic (1–2) or van der Waals interactions (3). Moreover, complex/DNA-binding experiments indicate that complexes 2 and 3 interact weakly with DNA, while no interaction is observed between complex 1 and DNA, probably due to the repulsion involving the free carboxylate group/DNA-phosphate. Anti-Mycobacterium tuberculosis (MTB) activity and cytotoxicity assays against one normal cell line V79 (hamster fibroblast) and three human cancer cell lines A549 (lung), MCF7 and MDA-MB-231 (breast) revealed that complexes 2 and 3 exhibit good activity against MTB and tumor cells, presenting high cytotoxicity (low IC50). On the other hand, complex 1 is practically inactive. Therefore, the best biological results found for complex 2 can be attributed to its esterification, improving the lipophilicity and cellular uptake, in order to facilitate its passive permeation through the tumor cell membranes allowing for cell death, as well as DNA and HSA interactions, when compared with complex 1.


 Please wait while we load your content...
Please wait while we load your content...