Synthesis of block copolymers using poly(methyl methacrylate) with unsaturated chain end through kinetic studies†
Abstract
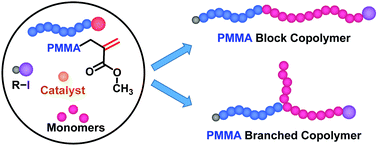
A poly(methyl methacrylate) (PMMA) with an unsaturated chain end (PMMA–Y) was used as a macroinitiator in the polymerizations of several monomers to generate block copolymers via addition–fragmentation chain transfer (AFCT). PMMA–Y also worked as a macromonomer to generate branched polymers via propagation. A kinetic study revealed that the occurrence of AFCT and propagation significantly depends on temperature in the styrene polymerization; namely, while propagation was predominant below 60 °C as previously reported, AFCT was predominant at elevated temperatures such as 120 °C as newly revealed in the present work. This new kinetic finding opened up an efficient synthesis of block copolymers of PMMA with polystyrene at an elevated temperature. AFCT was also predominant over propagation in the polymerizations of acrylonitrile and acrylates. Thus, block copolymers of PMMA with polyacrylonitrile and functional polyacrylates were successfully obtained. The polymerization was controlled using iodine transfer polymerization (ITP) for styrene and reversible complexation mediated polymerization (RCMP) for the other monomers. PMMA–Y with different molecular weights were also tested. This approach to obtain block copolymers is practically attractive for the ease of operation.


 Please wait while we load your content...
Please wait while we load your content...