Catalyst-free, aza-Michael polymerization of hydrazides: polymerizability, kinetics, and mechanistic origin of an α-effect†
Abstract
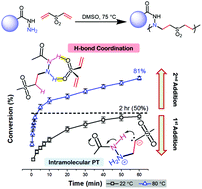
Despite the powerful nature of the aza-Michael reaction for generating C–N linkages and bioactive moieties, the bis-Michael addition of 1° amines remains ineffective for the synthesis of functional, step-growth polymers due to the drastic reduction in reactivity of the resulting 2° amine mono-addition adduct. In this study, a wide range of commercial hydrazides are shown to effectively undergo the bis-Michael reaction with divinyl sulfone (DVS) and 1,6-hexanediol diacrylate (HDA) under catalyst-free, thermal conditions to afford moderate to high molecular weight polymers with Mn = 3.8–34.5 kg mol−1. The hydrazide-Michael reactions exhibit two distinctive, conversion-dependent kinetic regimes that are 2nd-order overall, in contrast to the 3rd-order nature of amines previously reported. The mono-addition rate constant was found to be 37-fold greater than that of the bis-addition at 80 °C for the reaction between benzhydrazide and DVS. A significant majority (12 of 15) of the hydrazide derivatives used here show excellent bis-Michael reactivity and achieve >97% conversions after 5 days. This behavior is consistent with calculations that show minimal variance of electron density on the N-nucleophile among the derivatives studied. Reactivity differences between hydrazides and hexylamine are also explored. Overall, the difference in reactivity between hydrazides and amines is attributed to the adjacent nitrogen atom in hydrazides that acts as an efficient hydrogen-bond donor that facilitates intramolecular proton-transfer following the formation of the zwitterion intermediate. This effect not only activates the Michael acceptor but also coordinates with additional Michael acceptors to form an intermolecular reactant complex.


 Please wait while we load your content...
Please wait while we load your content...