Polymerization of 1-chloro-2-phenylacetylene derivatives by using a Brookhart-type catalyst†
Abstract
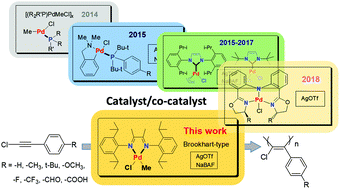
We report the polymerization of a series of disubstituted acetylenes including nonpolar and highly polar groups by a Brookhart-type α-diimine methyl palladium catalyst [(α-diimine)PdMeCl] with high molecular weight for the first time. 1-Chloro-2-(para-substituted)-phenylacetylenes with a highly polar carboxy group were polymerized in the polar solvent tetrahydrofuran by the combination of (α-diimine)PdMeCl and silver trifluoromethyl sulfonate (AgOTf), affording a molecular weight of 27 400 and a narrow polydispersity index of 1.12. Both the AgOTf and sodium tetrakis[3,5-bis-(trifluoro-methyl)phenyl]borate (NaBAF) are workable cocatalysts for the polymerization of 1-chloro-2-(para-substituted)-phenylacetylenes; the activity of the (α-diimine)PdMeCl/AgOTf catalyst system is higher than that of (α-diimine)PdMeCl/NaBAF under the same reaction conditions. The present work has enlarged the application range of the Brookhart-type catalyst and the types of polymerizable disubstituted acetylenes.


 Please wait while we load your content...
Please wait while we load your content...