Synthesis of telechelic polyesters by means of transesterification of an A2 + B2 polycondensation-derived cyclic polyester with a functionalized diester†
Abstract
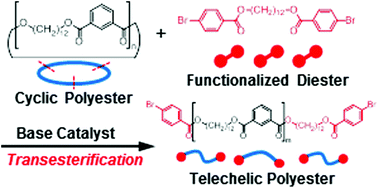
End-functionalized linear polyesters (LPEs) were synthesized by means of base-catalyzed transesterification of a cyclic polyester (CPEs), obtained by A2 + B2 polycondensation, with a symmetric functional diester as an exchange reagent (ExR). Among the metal alkoxides examined, tBuOK was the most effective for transesterification of cyclic poly(dodecamethylene isophthalate) (CPEs-1) with dodecamethylene bis(4-bromobenzoate) (ExR-1) at room temperature, affording LPEs with bromophenyl ends (LPEs-1-BrPh). However, CPEs-1 in the lower-molecular-weight region remained even after 95 h. Examination of the time course of the transesterification of CPEs-1 with ExR-1 and the reaction of LPEs with tBuOK revealed that the transesterification reached equilibrium between LPEs and CPEs within 1 min, and that the remaining CPEs-1 in the lower-molecular-weight region represented not the starting materials but the equilibrium products. Accordingly, the transesterification was carried out at a higher concentration to obtain LPEs-1-BrPh selectively, while the formation of CPEs was suppressed.


 Please wait while we load your content...
Please wait while we load your content...