Multi-olefin containing polyethers and triazolinediones: a powerful alliance†
Abstract
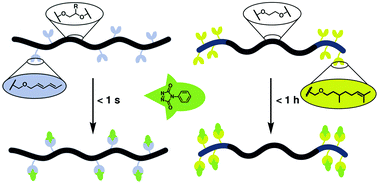
Multi-functional polyethers with ene or diene moieties were prepared via the polymerisation of tailored functional glycidyl ether monomers to create a platform for click chemistry with triazolinediones (TADs). Specifically, two novel monomers (i) 2,4-hexadien-1-yl glycidyl ether (HDEGE) and (ii) citronellyl glycidyl ether (CitroGE), designed to undergo a Diels–Alder or Alder–Ene reaction with TADs, respectively, were synthesized and subsequently transformed into a variety of tailored polyethers, either via the monomer activated method or anionic ring opening polymerisation. Multi-functional copolymers of HDEGE with ethylene oxide, propylene oxide and ethoxy ethyl glycidyl ether as well as ABA and AB poly(ethylene glycol)-based block copolymers with CitroGE are reported to demonstrate the scope of this platform. The ene and diene moieties were reacted with both mono- and bis-funtional TAD compounds to yield quantitative and irreversible modification with adjustable reaction kinetics, ranging from seconds for the HDEGE-based polymers to several minutes for the CitroGE-based polymers. In case of bis-functional TAD compounds, network formation and rapid gelation were achieved. The herein described multi-functional polyethers based on HDEGE and CitroGE are introduced as a platform to enable facile, fast, metal-free and quantitative functionalization of polyethers under mild conditions with an adjustable reaction time span.


 Please wait while we load your content...
Please wait while we load your content...