A synthetic strategy toward isosorbide polycarbonate with a high molecular weight: the effect of intermolecular hydrogen bonding between isosorbide and metal chlorides†
Abstract
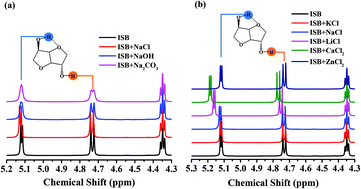
Isosorbide polycarbonate (ISB-PC) was prepared by melt transesterification and polycondensation reaction by employing ISB and diphenyl carbonate (DPC) as monomers. The effects of catalyst on the reactivity and the reactivity disparity of two hydroxyl groups (endo-OH and exo-OH) on ISB were investigated. The result shows that a metal chloride can form intermolecular hydrogen bonding with ISB hydroxyl groups, and the ISB intramolecular hydrogen bonding facilitates a stronger intermolecular hydrogen bonding between endo-OH and the catalyst. The intermolecular hydrogen bonding is capable of improving preferentially the endo-OH reactivity and thus increases the content of the endo–endo (a1) structure on the ISB-PC chain, resulting in a high Tg of the product. Further balancing of the reactivity disparity between endo-OH and exo-OH can be achieved by adjusting log β1 of the alkali metal chloride. It is found that the lithium chloride (LiCl) catalyst can significantly reduce the reactivity disparity, and the viscosity-average molecular weight of the synthesized ISB-PC reaches 51 000 g mol−1.


 Please wait while we load your content...
Please wait while we load your content...