Installing a functional group into the inactive ω-chain end of PMMA and PS-b-PMMA by terminal-selective transesterification†
Abstract
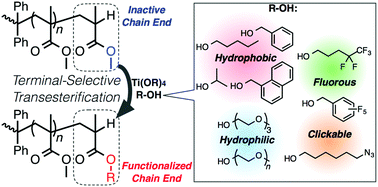
Polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA) has been widely used as a nanotemplate material for the block copolymer lithography. Thus, development of a simple method to functionalize the inherently inactive PS-b-PMMA is of considerable interest to expand its potential applications. Herein, we demonstrate that titanium alkoxide-catalyzed transesterification can be a versatile chain-end functionalization method for proton-terminated PS-b-PMMA. Starting from anionically synthesized PMMA and PS-b-PMMA, the ester-side chain of the MMA unit at the ω-chain end was selectively transesterified in the presence of various alcohols with the aid of the corresponding titanium alkoxides to yield the end-functionalized PMMAs and PS-b-PMMAs. A wide range of alcohols containing various functional groups (e.g., alkyl chains, aromatic rings, and fluorocarbon chains) were utilized for terminal-selective transesterification. Clickable groups (e.g., pentafluorobenzyl and azido) were also successfully incorporated into the ω-terminal MMA unit, which serves as the reactive site for further modification. Importantly, even the hydroxy-functionalized polymer poly(ethylene glycol) monomethyl ether could be used for the terminal-selective transesterification of PS-b-PMMA, yielding a polystyrene-block-poly(methyl methacrylate)-block-poly(ethylene glycol) triblock terpolymer. The present transesterification system can be a powerful tool for chain-end functionalization of the inactive chain-end of PS-b-PMMA, providing insight into the effect of the chain-end structure on microphase separation behavior.


 Please wait while we load your content...
Please wait while we load your content...