Tuning PNIPAm self-assembly and thermoresponse: roles of hydrophobic end-groups and hydrophilic comonomer†
Abstract
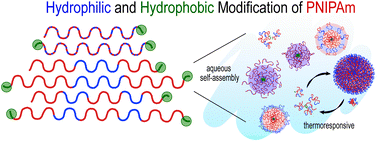
Modifications to the aqueous solution self-assembly and thermoresponsive properties of poly(N-isopropylacrylamide) (PNIPAm) can be achieved by hydrophobic end-group functionalization and incorporation of hydrophilic N,N-dimethylacrylamide (DMA) repeat units. Although these variations have been studied separately in the past, the simultaneous effects of both modifications have not been investigated systematically. Herein, we report the synthesis of six NIPAM and DMA based statistical, ABA triblock, and ABABA pentablock copolymers using reversible addition–fragmentation chain transfer (RAFT) polymerization, each containing one or two dodecyl hydrocarbon end-groups. Assembly into nanoscale particles and thermoresponsive properties in phosphate buffered saline were studied using light scattering and diffusion ordered NMR spectroscopy. Cloud points (Tcp) remained between 30–45 °C, notwithstanding hydrophobic modification. Copolymers with two alkyl tails assembled into flower-like micelles below the Tcp. The monofunctional statistical copolymer formed a core–shell assembly and the monofunctional ABA and ABABA polymers were molecularly dissolved. Above the Tcp, reversible precipitation was observed in all systems except for the monofunctional ABABA pentablock, which unexpectedly formed uniform large (>100 nm diameter) aggregates interpreted as mesoglobules. These results demonstrate surprising and delicately balanced tradeoffs between short non-polar end groups and tailored hydrophobicity in the nanoscale self-assembly of PNIPAm based copolymers in water near the lower critical solution temperature.


 Please wait while we load your content...
Please wait while we load your content...