Bromoalkyl ATRP initiator activation by inorganic salts: experiments and computations†
Abstract
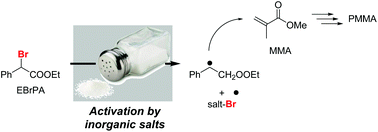
Ethyl α-bromophenylacetate (EBrPA) is able to initiate the bulk radical polymerisation of methyl methacrylate (MMA) under thermal conditions (90 °C) in the presence of a variety of simple alkali or alkaline-earth metal or nBu4N salts (Mt+X− with X− = chloride, bromide, iodide, carbonate, bicarbonate, sulfate, bisulfate, nitrate, hydroxide and hexafluorophosphate). Chain growth is controlled only when using iodide salts, which also gives one of the highest polymerisation rates. Using a substoichiometric amount of LiI as an activator, the polymerisation rate is unaffected by the LiI/initiator ratio when the initiator is cyanoisopropyl iodide (CPI) but increases with the activator amount when using EBrPA. Analysis of these rates in combination with the polymer molecular weights revealed a LiBr-catalysed halogen exchange between more active PMMA-I and less active PMMA-Br chains. In combination with a computational investigation by DFT methods, these findings demonstrate that while the rate of polymerisation is determined by the atom transfer equilibrium from EBrPA to the Mt+X− catalyst to yield EPA˙ and Mt+(BrX˙)−, controlled chain growth cannot be ensured exclusively by the persistent radical effect.


 Please wait while we load your content...
Please wait while we load your content...