Design of photoinitiator-functionalized hydrophilic nanogels with uniform size and excellent biocompatibility†
Abstract
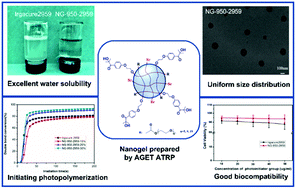
Three hydrophilic photoinitiator-functionalized nanogels with uniform size distribution were designed and synthesized through activator generated electron transfer atom transfer radical polymerization (AGET ATRP) in an inverse miniemulsion, based on oligo(ethylene glycol) monomethyl ether methacrylate (OEOMA), polyethylene glycol dimethacrylate (PEGDMA) and 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959). Compared to Irgacure 2959, the nanogels possess excellent water solubility which reaches up 50 wt%. The nanogels have an absorption centered at 273 nm in anhydrous acetonitrile and can effectively initiate the polymerization of acrylate monomers. The nanogels can not only improve the thermostability of the polymer, but can also exhibit an excellent toughening effect. Furthermore, the migration of photolysis fragments from a photocured film was alleviated significantly due to their high molecular weight. Importantly, the cytotoxicity of the nanogels and their photocured film was tested against HeLa cervical cancer cells using the MTT cell viability assay and their cell viability is 93%–94%, indicating that the nanogels have potential in biomaterials.


 Please wait while we load your content...
Please wait while we load your content...