A substituent para-to-ortho positioning effect drives the photoreactivity of a dibenzothiophene-based oxalate series used as LED-excitable free radical photoinitiators†
Abstract
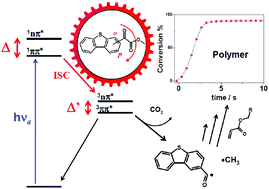
Four dibenzothiophene (DBT)-based methyl oxalates, synthesized through a one-step Friedel–Crafts acylation reaction, were developed as high-performance photoinitiators (PIs) for the free radical polymerization of acrylate resins excitable using LED sources that emit in the near-UV and visible region (i.e. 365–425 nm). These type I PIs show good optical absorption properties (ε365 nm = 1 000–5300 M−1 cm−1). Their respective photophysical properties, as well as the sequential photocleavage mechanism, were investigated using a large set of theoretical and experimental methods. A relevant structure/reactivity relationship opening the way to enhancing the PI performance is highlighted. Indeed, a para-to-ortho positioning effect of the oxalate-based substituent regulates both the absorption and photocleavage abilities within this DBT series. Para-isomers consequently exhibit a photoinitiating reactivity, whose efficiency is more than one order of magnitude higher than that of their ortho homologues. Associated with a H-donor alkylamine co-reactant, these photoinitiating efficiencies can be amplified even more due to an additional reaction pathway that judiciously assigns a new role to the aryloyl radical by-products. These results correlate the PI photopolymerization performance with the photoinduced processes occurring in the excited states.


 Please wait while we load your content...
Please wait while we load your content...