Bisdithiooxalate as novel coupling agent for amino-terminated polyamides†
Abstract
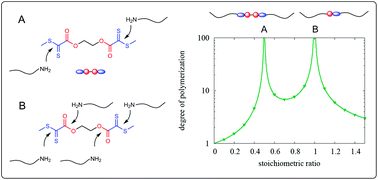
Bisdithiooxalate has recently been introduced as a new thermo-reversible hetero Diels–Alder dilinker. Here, we demonstrate that the same compound has proved to be a very effective coupling agent for amino-terminated polyamides. Model reactions with aliphatic amines in solution show that bisdithiooxalate possesses four reactive centers which already react considerably fast at low temperatures (−10 °C). Under consideration of all assumed immediate and subsequent reactions, a kinetic model with four partial reactions is set up which allows to approximate the development of molar masses during chain extension of polyamide 12 theoretically. According to the model, high molar masses are achieved in a relatively wide range of mixing stoichiometry (ratio of coupling agent to amino-terminal groups). This is an essential advantage over conventional bifunctional coupling agents which require equivalency of the reacting groups to achieve high molar masses. In fact, coupling reactions of amino-terminated polyamide 12 in the melt show extraordinary increases in molar mass in a very short period of time over a wide concentration range. The key reactions of the coupling agent were identified by NMR spectroscopy. Deviations of the coupling behavior from the theoretical expectations are attributed to insufficient mixing of the components in the melt, which was not taken into account in the model.


 Please wait while we load your content...
Please wait while we load your content...