Understanding the ring-opening polymerisation of dioxolanones†
Abstract
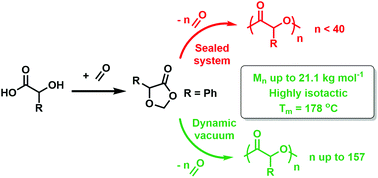
Sustainable monomers built from ring-closed α-hydroxyacids to form 1,3-dioxolane-4-ones (DOX) are both easily prepared and functional group tolerant. Subsequent ring-opening polymerisation (ROP) of DOX furnishes a broad scope of functional poly(α-hydroxy acid)s (PAHAs). Elusive polymers like isotactic poly(mandelic acid) are accessible and we now report that the formaldehyde eliminated during the polymerisation can induce a competing side reaction. In this contribution, we propose a new mechanism of ring-opening for these monomers, involving competitive elimination and a subsequent Tishchenko reaction facilitated by formaldehyde. Both catalyst design and polymerisation methodology can be modified to reduce the impact of the Tishchenko reaction, with sterically-unencumbered and electronically-neutral salen aluminium catalysts exhibiting the best performance in the ROP of 5-phenyl-1,3-dioxolane-4-one (PhDOX), providing the best balance of reactivity and selectivity. Importantly, crystalline poly(mandelic acid) was obtained using a dynamic vacuum ROP either neat or in diphenyl ether, where volatilisation of the formaldehyde allows for production of polymers with thermal properties competitive with commercial polystyrene.
- This article is part of the themed collection: Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...