Tetraphenylethene-decorated functional polybenzoxazines: post-polymerization synthesis via benzoxazine–isocyanide chemistry and application in probing and catalyst fields†
Abstract
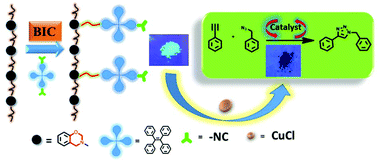
Benzoxazines have been widely studied as thermosetting plastics; in contrast, scarce attention has been focused on their functionalization. In this effort, a series of tetraphenylethene (TPE)-decorated, functional polybenzoxazines were successfully constructed by post-polymerization of a main-chain type benzoxazine precursor (P1) via efficient benzoxazine–isocyanide chemistry (BIC) under mild conditions. The obtained polymers were characterized by FT-IR, 1H NMR and size exclusion chromatography (SEC) analyses. The representative polymer, P2-2, exhibits typical aggregation-induced emission enhancement (AIEE) characteristics, and was applied as a fluorescent probe to detect nitro compounds in high-water fraction medium (fw% = 90%). In addition, the residual isocyano (–NC) groups on the obtained polymer (P2-3) were utilized to bind with CuCl, and the resulting polymer/metal complex (P2-3-CuCl) can be applied as an efficient heterogeneous catalyst for an azide–alkyne cycloaddition reaction in air. This current work provides a simple and effective method for the functionalization of easily obtained polybenzoxazines to further expand their application scope.


 Please wait while we load your content...
Please wait while we load your content...