The influence of stereochemistry on the reactivity of the Diels–Alder cycloaddition and the implications for reversible network polymerization†
Abstract
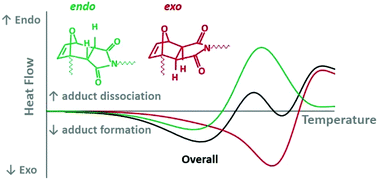
A detailed calorimetric investigation is performed on the influence of the stereochemistry of the furan–maleimide Diels–Alder reaction on the kinetics of the reversible network formation, in the absence of solvents. Two stereoisomers are formed, with the endo isomer forming kinetically faster and undergoing cycloreversion at lower temperatures than the more thermodynamically stable exo isomer. Rate constants and activation energies for the forward and retro Diels–Alder reaction of both stereoisomers were derived by modelling isothermal (microcalorimetry) and non-isothermal (DSC) data. The kinetic model was further verified using time-resolved and temperature-controlled 1H NMR spectroscopy. The influence of time, temperature, and the maleimide/furan ratio on the exo/endo ratio are presented. The implications of the formation of two stereoisomers on the thermoresponsiveness and thermal behaviour for thermal processing and self-healing applications are discussed.


 Please wait while we load your content...
Please wait while we load your content...