Synthesis of silver-loaded ZnO nanorods and their enhanced photocatalytic activity and photoconductivity study†
Abstract
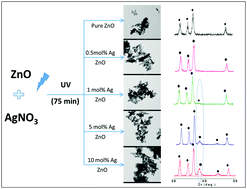
Herein, we synthesized ZnO nanorods using a solvothermal reaction technique at 200 °C for 24 h, and the prepared ZnO nanorods were decorated with silver (Ag) nanoparticles to enhance their photocatalytic activity. The Ag nanoparticles were photochemically deposited on the ZnO rods with varying molar concentrations (from 0.5 to 10 mol%), and their various physicochemical properties were studied. The prepared material was characterised using different spectroscopic techniques. XRD revealed the formation of a highly crystalline hexagonal phase of ZnO. For a higher silver loading (>5 mol%), separate peaks corresponding to cubic silver were observed in the XRD pattern. The photoluminescence spectra of the Ag/ZnO nanostructures show two distinct peaks at 390 and 500 nm; interestingly, the PL intensity of the ZnO emission peak at 500 nm decreases with an increase in the silver concentration. The diffuse reflectance spectra of Ag/ZnO indicate absorbance at 380 nm due to ZnO and a slight hump at 440 nm that corresponds to silver nanoparticles. The FE-SEM and TEM analysis indicates the formation of a hexagonal rod-like morphology, with the lengths of the rods ranging from around 50 to 200 nm and a diameter of around 30 nm. TEM also confirms the presence of Ag nanoparticles with sizes in the range of 20 to 30 nm on the surface of the ZnO nanorods. The photocatalytic activity of the Ag/ZnO nanostructures was evaluated by following the degradation of methylene blue (MB) dye under a 400 W mercury vapour lamp. ZnO with 10 mol% Ag loading shows the highest photocatalytic activity as compared to the 0.5, 1 & 5 mol% Ag–ZnO catalysts. The observed apparent rate constant for the photocatalytic MB degradation using 10 mol% Ag–ZnO (Kapp = 6.01 × 10−2 min−1) was six times that of pure ZnO (Kapp = 1.09 × 10−2 min−1). A gradual increase in the photocatalytic activity of Ag/ZnO was observed with an increase in the silver concentration. The photocurrent response of the prepared Ag–ZnO nanostructures was examined by a photoconductivity study. Moreover, the photocatalytic performance of the sample was correlated with the photoconductivity of the samples. The photoconductivity of the samples was stable, and the photoconductivity of 10 mol% Ag–ZnO was almost 20 times that of pure ZnO, resulting in a higher photocatalytic activity.


 Please wait while we load your content...
Please wait while we load your content...