Synthesis and vitamin D receptor affinity of 16-oxa vitamin D3 analogues†
Abstract
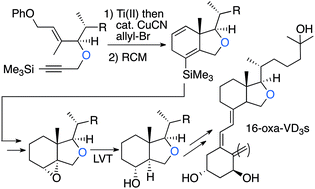
Two novel 16-oxa-vitamin D3 analogues were synthesized using a tandem Ti(II)-mediated enyne cyclization/Cu-catalyzed allylation, Ru-catalyzed ring-closing metathesis reaction, and a low-valent titanium (LVT)-mediated stereoselective radical reduction of 8α,14α-epoxide as the key steps for the synthesis of the 16-oxa-C,D ring unit. The vitamin D receptor-binding affinity of the synthesized analogues, 16-oxa-1α,25-(OH)2VD3 and 16-oxa-19-nor-1α,25-(OH)2VD3, was evaluated by fluorescence polarization vitamin D receptor competitor assay and time-resolved fluorescence energy transfer vitamin D receptor co-activator assay.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...